
Adhering to regulatory frameworks is the backbone of successful product packaging. It moves beyond aesthetic considerations to ensure consumer safety and legal marketability. Brands often underestimate the rigorous nature of labeling laws until they face a recall or a lawsuit.
You must understand that label compliance is dynamic. Regulations shift based on jurisdiction, product category, and distribution channels. A label that is compliant today may require revisions tomorrow due to updated FDA mandates or new state-level legislation.
This guide bypasses the rudimentary design advice to focus on the technical execution of compliant packaging. It addresses the intersection of legal obligation and supply chain transparency. Failure to audit these elements results in costly production delays and inventory waste.
Label compliance refers to the strict adherence to statutes governing the presentation of product information. It involves accurate representation of contents, safety warnings, and usage instructions. These standards are enforced by bodies like the FDA, FTC, and CPSC in the United States, along with international counterparts.
Compliance is binary. Your packaging is either legally sound or it constitutes misbranding. Misbranded products are subject to seizure, and the responsible entity may face steep fines. This definition encompasses everything from font size minima to the contrast ratio of barcodes.
Regulatory alignment ensures that the Principal Display Panel (PDP) and the Information Panel effectively communicate the product’s nature. It requires a deep understanding of the Code of Federal Regulations (CFR) applicable to your specific vertical.
This process also verifies that all marketing claims are substantiated. You cannot imply benefits that the product does not possess. Every adjective used on the packaging must withstand legal scrutiny to prevent allegations of false advertising or deceptive trade practices.
Compliance protects your bottom line. The cost of rectifying a labeling error after printing is exponential compared to the cost of pre-press verification. A single non-compliant phrase can trigger a nationwide recall, destroying profit margins for the fiscal year.
Retailers impose their own compliance standards. Major distributors and marketplaces like Amazon effectively audit your labels before listing. Non-compliance leads to suspension of vendor privileges, effectively cutting off your revenue stream until the issue is rectified.
Consumer trust relies on transparent labeling. In an era of heightened scrutiny regarding ingredients and sourcing, accuracy fosters loyalty. When a consumer spots a discrepancy or a hidden additive, the brand reputation suffers immediate, long-term damage.
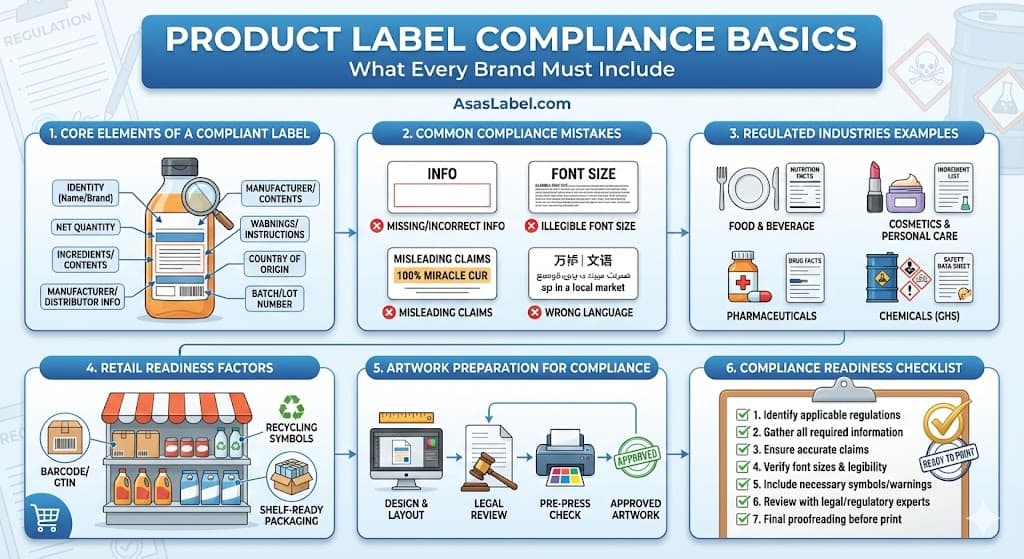
Regardless of the product category, certain data points remain non-negotiable. These core elements form the legal skeleton of your packaging design. They ensure the consumer knows exactly what they are buying and who is responsible for its production.
Federal regulations mandate specific placements for these elements. You cannot hide critical data in the folds of a package or on the bottom panel if regulations dictate otherwise. The Principal Display Panel serves as the primary real estate for consumer identification.
Designers must balance creative liberty with these mandatory inclusions. The text must be legible, typically requiring specific contrasting backgrounds. Understanding the hierarchy of these elements is crucial for passing regulatory audits.
The Statement of Identity allows consumers to recognize the commodity instantly. This is not the brand name nor the fanciful marketing name. It is the common or usual name of the product, such as "Shampoo" or "Tomato Sauce."
This statement must appear on the PDP in bold type. It should be one of the most dominant features, generally parallel to the base of the package. The size of the text must be reasonably related to the most prominent printed matter on the label.
Ambiguity here is a primary cause of rejection. If a product is a "Cheese Product" rather than "Cheese," the distinction is legal, not stylistic. Failing to declare the correct legal name constitutes misbranding and invites regulatory action.
The Net Quantity of Contents declaration tells the consumer how much product is in the container. This must be expressed in both metric (grams, liters) and U.S. Customary units (ounces, fluid ounces). Accuracy here prevents consumer deception regarding value.
You must place this declaration within the bottom 30% of the PDP. This area must be free of other graphic elements that crowd the text. The font size is determined by the surface area of the PDP, ensuring readability regardless of package size.
Batch and lot codes are essential for supply chain traceability. In the event of a contamination issue, these codes allow for targeted recalls rather than total inventory withdrawal. They serve as the critical link between the physical unit and your manufacturing records.
Expiration dates and "Best By" markings are category-dependent but crucial for perishable goods. These dates must be printed clearly and permanently. They facilitate inventory rotation for retailers and ensure the consumer experiences the product at peak quality.
The label must identify the responsible party. This section typically reads "Manufactured by," "Distributed by," or "Packed by." It establishes legal liability and provides a contact point for consumers and regulators.
This declaration must include the business name and mere street address. If the business is listed in a current telephone directory or online public database, the street address may sometimes be omitted, leaving just the city, state, and zip code.
Third-party manufacturing arrangements complicate this section. If you use a contract manufacturer, you may list your brand as the distributor. However, the qualifying phrase "Distributed by" must be used to maintain transparency regarding the product's origin.
Universal labeling rules provide a foundation, but vertical-specific mandates dictate the details. A label compliant for a detergent would be illegal for a beverage. You must consult the specific CFR titles relevant to your industry to ensure full compliance.
These distinctions often center on safety warnings and ingredient nomenclature. The intended use of the product defines which regulatory body holds jurisdiction. This classification determines the specific formatting requirements for your information panels.
Food labeling falls under NLEA and FDA jurisdiction. It requires a Nutrition Facts panel with specific formatting for calories, fats, and nutrients. Allergen declarations are mandatory and must follow strict syntax, such as "Contains: Milk, Soy."
Cosmetic labels adhere to the FD&C Act and FP&L Act. Ingredients must be listed in descending order of predominance using proper INCI specifically. Warning statements are required for items like pressurized containers or products containing specific alpha-hydroxy acids.
Chemical products, including household cleaners, often fall under CPSC or OSHA guidelines. They frequently require GHS compliant formatting. This includes specific pictograms, signal words like "DANGER" or "WARNING," and standardized hazard statements that cannot be altered.
Dietary supplements occupy a unique space. They utilize a "Supplement Facts" panel, which differs technically from Nutrition Facts. Disclaimer text regarding FDA evaluation is mandatory for any structure-function claims made on the packaging.
Compliance failures rarely stem from malice. They usually result from ignorance of technical nuances or lack of oversight during the design phase. Identifying common pitfalls helps you insulate your production process against expensive errors.
Design-first thinking often overrides regulatory logic. When aesthetics take precedence over legibility and mandatory placement, compliance is compromised. Reviewing the latest enforcement reports from agencies reveals repetitive patterns of negligence.
Missing allergen alerts is a top reason for recalls. If a facility processes peanuts, and the label fails to declare potential cross-contamination, the health risk is severe. This oversight is treated with zero tolerance by regulatory agencies.
Incorrect type size is a frequent technical error. The "1/16-inch rule" for lowercase letters is often violated on small packaging. If the text is physically too small to meet the statutory requirement, the product is technically misbranded regardless of content accuracy.
Global distribution complicates language requirements. Canada requires bilingual English and French labeling with equal prominence. Neglecting local language laws in export markets leads to immediate customs rejection or retail removal.
Compliance must be integrated into the design workflow, not treated as an afterthought. Establishing a robust Standard Operating Procedure (SOP) for label creation significantly reduces risk. This involves multiple checkpoints before a file reaches the printer.
Involve regulatory affairs professionals at the concept stage. They can flag problematic claims before a designer commits hours to a layout. This proactive approach streamlines the revision process and anchors the creative in legal reality.
Utilize automated inspection software during the proofing phase. Digital proofreading tools can compare artwork files against approved copy decks pixel-by-pixel. This technology catches minute discrepancies in spelling or font size that human eyes often miss.
Maintain strict version control. Label regulations change, and ingredients change. Ensure that your print vendor is working from the absolute latest approved file. Old versions must be systematically retired to prevent the accidental production of non-compliant packaging.
Before launching print production, execute a final, ruthless audit. This checklist validates that every element meets regulatory standards. Do not rely on memory; verify each point against the current regulations.
Confirm PDP Calculations. Measure the area of the Principal Display Panel. Verify that the Net Quantity declaration meets the minimum height requirement relative to this surface area. Ensure the Statement of Identity is bold and dominant.
Validate Ingredient Lists. Check the ingredient deck against the final formulation sheet. Ensure proper nomenclature (INCI for cosmetics, common names for food). Verify the order of predominance is correct by weight.
Audit Claims and Marketing Copy. Review every adjective. Can you substantiate "All Natural"? Is "Organic" supported by a certifying body? Ensure no cosmetic products are making drug claims that imply curing or treating disease.
Check Contact Information. Verify the manufacturer or distributor address is current. Ensure the "Qualifying Phrase" (e.g., Manufactured for) is present if the brand is not the manufacturer. Test the layout for legibility.
Review Warning Labels. Ensure all safety warnings, flammability alerts, or choking hazards are present. Check that signal words use the correct font height and are encased in the mandatory border styles if applicable.
Assess Print Imperatives. Check "Quiet Zones" around barcodes to ensure scanability. Verify color contrast ratios for text against the background. Confirm that bleed and safe zones are respected to prevent information cut-off.